Drug development is a long and costly process that takes on average 10-15 years from the initial phase to final approval. Among 90% of drug development fails, almost half happens because of a lack of clinical efficacy.
To minimize the risks of failure, it’s important to ensure the best quality of clinical trials. Organizations achieve this through the implementation of clinical trial management software that helps streamline clinical workflows.
If you’re a vendor or provider aimed at improving data collection, trial planning, and supply management with the help of CTMS solutions, keep reading. You’ll discover how to distinguish various clinical trial systems and select the one that aligns with your healthcare needs.
Software for Clinical Trials: Short Overview
Clinical trial management software is a comprehensive technology platform designed to streamline the planning, execution, and management of clinical trials. It serves as a central hub for organizing and managing all aspects of a clinical trial, from patient recruitment to data collection, monitoring, and reporting.
Clinical trial management software facilitates the management of trial activities, ensuring compliance with regulatory standards and improving collaboration among trial stakeholders.
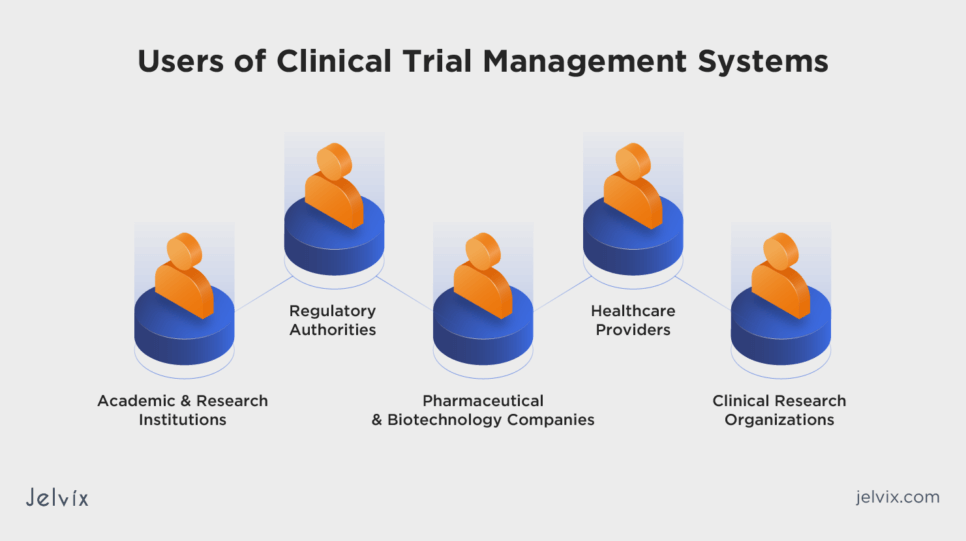
Who Uses Clinical Trial Management Systems
CTMS is used by clinical trial sponsors, research organizations, and healthcare institutions to efficiently plan, conduct, and manage clinical trials. These systems enable streamlined collaboration among researchers, monitor trial progress, and ensure regulatory compliance.
Pharmaceutical and Biotechnology Companies
These entities rely on CTMS to manage the complex logistics of developing new drugs and therapies, ensuring efficient trial conduct and compliance with regulatory requirements.
Clinical Research Organizations
CROs, which conduct clinical trials on behalf of sponsors, use CTMS to streamline trial processes, manage multiple trials simultaneously, and maintain high standards of data integrity and patient safety.
Academic and Research Institutions
Researchers and clinicians at healthcare institutions use CTMS to manage investigator-initiated trials, facilitating data management, regulatory compliance, and collaboration among research teams.
Healthcare Providers
Recruiting and retaining patients for clinical trials remains an ongoing challenge. As reported by NIH, up to 85% of trials fail to enroll enough patients. CTMS systems help hospitals and clinics involved in clinical trials manage patient recruitment more efficiently, ensuring accurate data collection and reporting.
Regulatory Authorities
CTMS assists regulatory authorities, such as the FDA and EMA, in monitoring trial progress, evaluating safety and efficiency, and ensuring that trials comply with regulatory standards and guidelines. This helps regulatory bodies make informed decisions regarding drug approvals and patient safety.
Clinical Trial Management Software Functionality
Clinical trial management software, such as EDC, CTMS, RTSM, and IRT, can offer a broad range of functionalities tailored to streamline the management and operational process in clinical trials. These features allow addressing specific challenges in clinical trial management to improve efficiency, data integrity, and compliance with regulatory standards:
- Trial planning and design: facilitating the creation and management of study protocols, ensuring clear objectives and methodologies are in place;
- Site and investigator selection: assisting in choosing optimal sites and investigators based on specific criteria, such as previous performance and patient demographics;
- Patient recruitment: enhancing the efficiency of patient screening and enrollment processes, including tracking recruitment progress and managing consent documentation;
- Visit scheduling and management: automating the scheduling of patient visits according to the study protocol and improving adherence to study timelines;
- Adverse event reporting: streamlining the reporting process for adverse events to ensure patient safety and regulatory compliance;
- Site monitoring: providing tools for monitoring trial sites for adherence to study protocols and compliance with Good Clinical Practice guidelines;
- Regulatory compliance: managing regulatory submissions and ensuring compliance with local and international regulations;
- Financial management: offering budgeting, financial tracking, and billing automation for managing the financial aspects of trials;
- Analysis and reporting: supporting the statistical analysis of trial data and generating reports for internal and regulatory review;
- Communication: offering a centralized platform for communication and document sharing among all trial stakeholders.
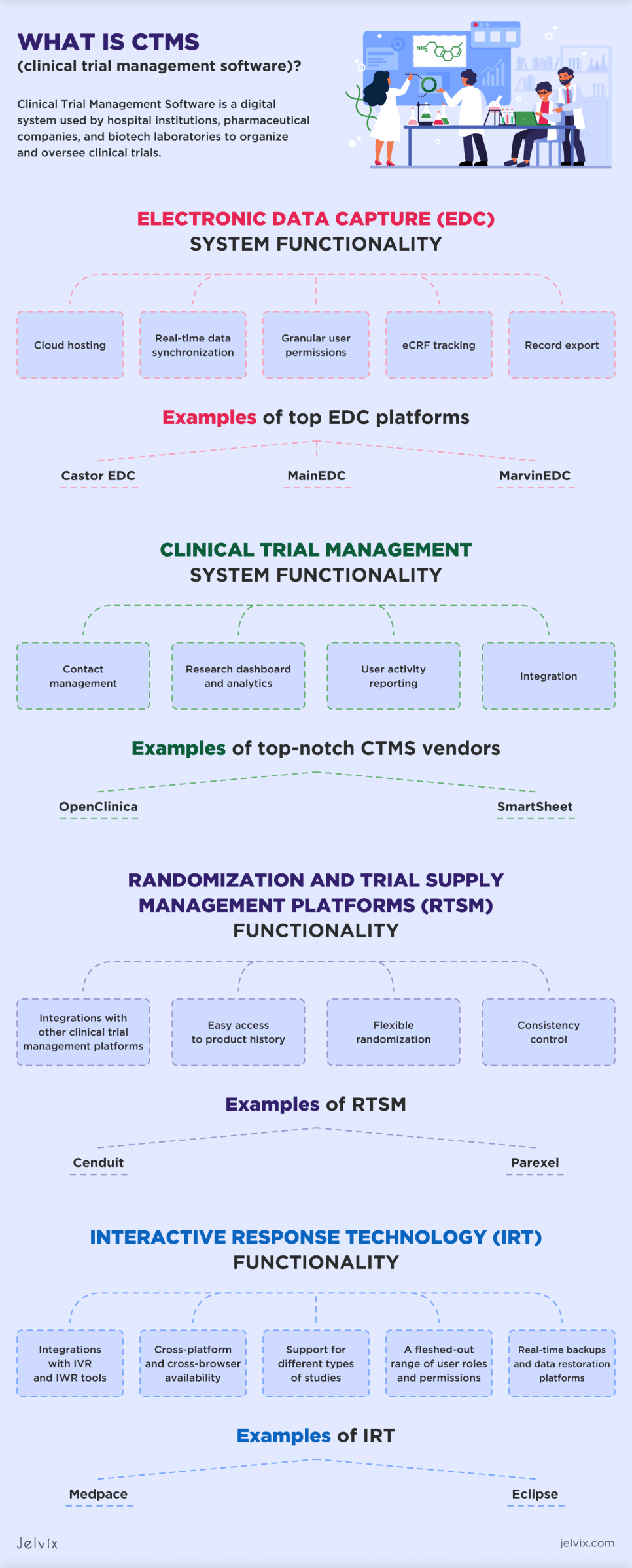
Electronic Data Capture System (EDC)
Electronic data capture is a solution used in clinical research to collect, manage, and store data generated during clinical trials and research studies. It replaces traditional paper-based methods of data collection, making the process more efficient, accurate, and secure. EDC systems allow researchers to enter, validate, and analyze data electronically, streamlining its management and reducing the likelihood of errors.
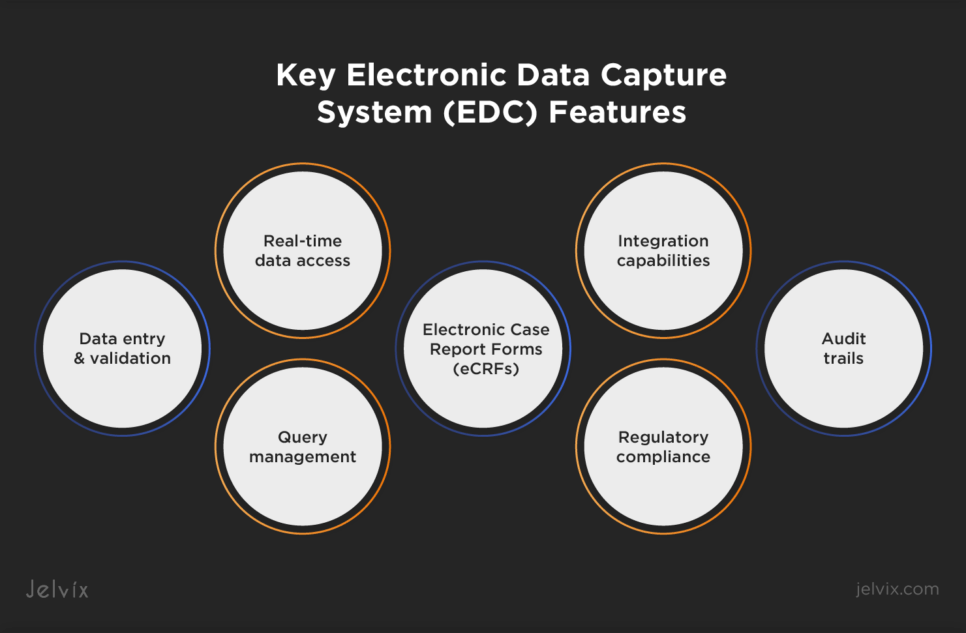
Key EDC Features
EDC systems come with a range of functionalities designed to optimize clinical trial processes:
- Data entry and validation: enables electronic entry of clinical trial data directly into the system. It includes built-in validation rules to ensure data accuracy and consistency;
- Query management: automatically generates queries for missing and inconsistent data, facilitating quick resolution and clean data for analysis;
- Electronic Case Report Forms (eCRFs): allow researchers to collect data electronically, eliminating the need for paper forms. Data can be entered directly into the system, reducing the risk of errors associated with manual data transcription;
- Real-time data access: provides investigators and trial managers with immediate access to data, enabling timely decision-making and remote patient monitoring;
- Audit trails: maintains a detailed record of all data changes, providing transparency and ensuring data integrity for regulatory compliance;
- Regulatory compliance: ensures compliance with regulatory guidelines such as 21 CFR Part 11, which covers electronic records and electronic signatures;
- Integration capabilities: offers integration with other clinical trial management tools and systems for seamless data exchange and workflow efficiency.
Examples of EDC Platforms
Several EDC platforms have gained prominence in the clinical research industry, each offering unique features and capabilities to meet the diverse needs of clinical trials.
Facilitating Data Reporting
Rave EDC by Medidata is a widely used solution that supports complex clinical trials across various phases and therapeutic areas. It ensures global compliance with regulatory standards and facilitates seamless integration with other clinical systems.
Smart Study Management
As part of Oracle’s Health Sciences suite, Oracle Clinical provides a secure environment for data management with advanced analytics. It supports a wide range of clinical trials, offering tools for effective data management and adherence to regulatory requirements.
Efficient Data Collecting
REDCap is a web-based application designed for database and survey creation. It emphasizes data security, project management, and user-defined workflows, supporting a diverse array of research studies with its customizable data collection tools.
What Is a Clinical Trial Management System (CTMS)?
A clinical trial management system (CTMS) is a comprehensive solution designed to streamline the planning, execution, and management of clinical trials. A CTMS facilitates centralizing trial data, improving recruitment and enrollment strategies, managing study budgets and finances, and ensuring compliance with regulatory requirements.
It provides tools for document management, scheduling, participant tracking, and data analysis, helping research organizations maintain accurate records, manage resources effectively, and make informed decisions.
Functionality of Clinical Trial Management Systems
The core functionalities of a CTMS include comprehensive planning, tracking, and management of clinical trials, including tasks like protocol design, patient enrollment, data collection, and regulatory compliance:
Contact management: offers centralized secure storage for all patient and sponsor data and enhances collaboration across teams by incorporating features for contact search, managing addresses, and importing data in batches;
Research dashboard and analytics: presents a visually engaging overview of a study’s progress. With the help of customizable reports and real-time notifications, researchers can monitor important milestones and gain valuable insights that can enhance the efficiency of future studies;
User activity tracking: involves monitoring and recording the actions and interactions of users within the system, providing insights into user engagement;
Integration capabilities: offers integration with big data platforms, analytics tools, and EDCs, facilitating broader data analysis and management.
Clinical Trial Management System Examples
Getting updated on top CTMS examples enables organizations to make informed decisions that align with their clinical trial objectives.
Managing Clinical Research
Tailored for site, enterprise, and network research operations, Bio-Optronics Clinical Conductor focuses on optimizing clinical trial operations, enhancing productivity, and improving patient recruitment and retention.
Customizing Data Management
MedNet Solutions is a customizable CTMS platform that can help you support the unique workflows of your healthcare organization. If you seek features for electronic document management, study planning, and progress tracking, alongside integrations with EDC systems for comprehensive data management, consider MedNet for your clinic.
Combining CTMS and QMS
If you want to combine CTMS capabilities with QMS (quality management system), consider implementing Dot Compliance. It allows stakeholders to effectively manage and monitor the entire clinical research and trial process through a centralized platform. This integration facilitates seamless access and collaboration on study information, Electronic Trial Master Files (ETMF), clinical documents, and tasks.
If you seek to enhance your clinical workflows with CTMS, contact our team. Jelvix experts will help you understand what’s needed to build a custom solution or integrate a ready-made one.
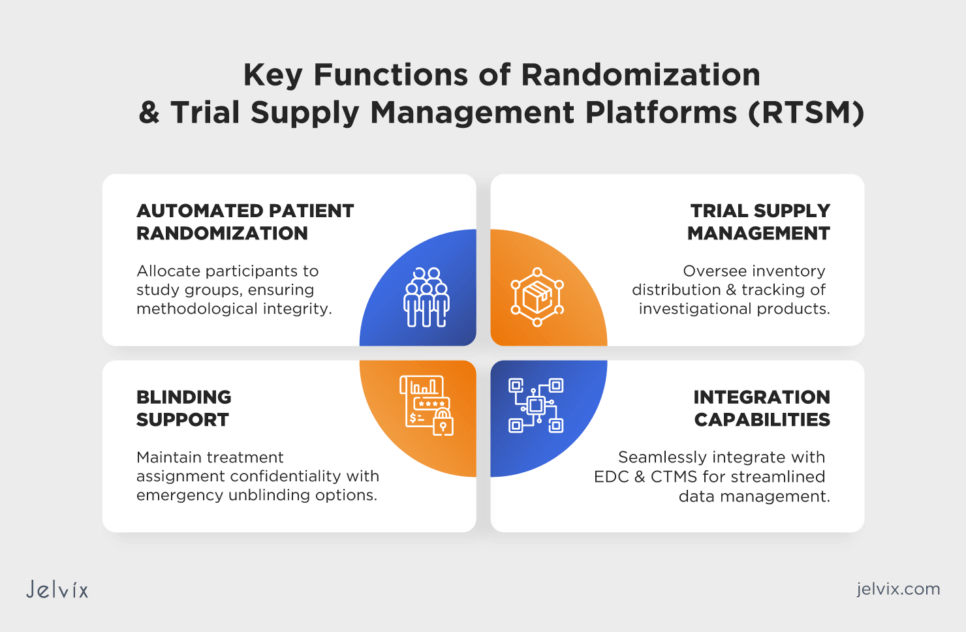
Randomization and Trial Supply Management Platforms (RTSM)
RTSM platforms ensure random allocation of patients to different treatment groups and manage the supply of investigational products. These systems are designed to support the blinding and unblinding processes, ensuring the integrity and confidentiality of the trial data. By automating the assignment of patients to groups, these platforms make the process more reliable, quick, and accurate.
Core Functionality of RTSM Systems
An RTSM platform offers a range of functionalities designed to streamline the clinical trial processes, such as:
- Automated patient randomization: facilitates the random allocation of participants to different study groups, ensuring the trial’s methodological soundness;
- Trial supply management: manages the inventory, distribution, and tracking of investigational products, ensuring that researchers have an adequate supply without overstocking or shortage;
- Blinding and unblinding support: maintains the confidentiality of treatment assignments, with secure mechanisms for emergency unblinding if necessary;
- Integration capabilities: seamlessly integrates with other clinical trial management tools, including EDC and CTMS, for efficient data management and analysis.
Examples of RTSM Platforms
Understanding the features and capabilities of different RTSM platforms allows research teams to choose the most suitable system that meets their specific needs. By examining various RTSM platforms, organizations can compare costs and potential return on investment, ensuring that they select a cost-effective solution that delivers value through streamlined operations and resource optimization.
Managing Complex Trial Designs
Prancer RTSM offers a wide range of features and robust functionality to support both complex and simple clinical trial designs. It provides the flexibility to adapt to various trial needs and scales effectively for global supply strategies. Prancer RTSM can be configured to align with the unique requirements of individual clinical trials and the specific business processes of organizations involved in clinical research.
Solving Oncology Trial Challenges
Cenduit enhances randomization processes by offering an extensive array of built-in methods and real-time cohort management. Researchers gain access to multiple randomization options, including simple, blocked, and unequal, providing flexibility in clinical trial management.
Enhancing Trial Delivery
Clario RTSM offers a wide range of features and capabilities, including randomization methods, inventory management, patient enrollment, and real-time data tracking. It helps clinical trial professionals streamline their processes, ensure accurate allocation of treatments, and maintain control over the trial’s drug supply chain.
Interactive Response Technology (IRT)
Interactive response technology is a critical component of clinical trial management. It is used in clinical trial management to facilitate randomization, patient enrollment, drug supply management, and data tracking. It allows clinical trial teams to manage various aspects of the trial efficiently, ensuring proper allocation of treatments, accurate data collection, and seamless communication between stakeholders.
Key Functionality of IRTs
Core features of interactive response technologies include:
- Integrations with IVR and IWR: IRTs seamlessly integrate with interactive voice response and interactive web response tools to enable efficient communication and data collection during clinical trials;
- Cross-platform and cross-browser availability: IRTs are accessible across multiple platforms, including desktop and mobile devices, and support various web browsers ensuring flexibility and accessibility for users;
- Versatility for studies: IRTs offer support for a wide range of study types, including extension studies, sequencing studies, sub-studies, and crossover studies. This versatility allows clinical trial managers to design and manage various trial formats effectively;
- User roles and permissions: IRT platforms provide a comprehensive range of user roles and permissions, enabling precise control over access and data security;
- Real-time backups and data restoration: IRT systems offer real-time backup mechanisms and data restoration platforms to ensure data integrity and provide efficient disaster recovery capabilities.
Let's examine the computerized provider order entry definition, evaluate its pros and cons, and determine strategies for successful implementation.
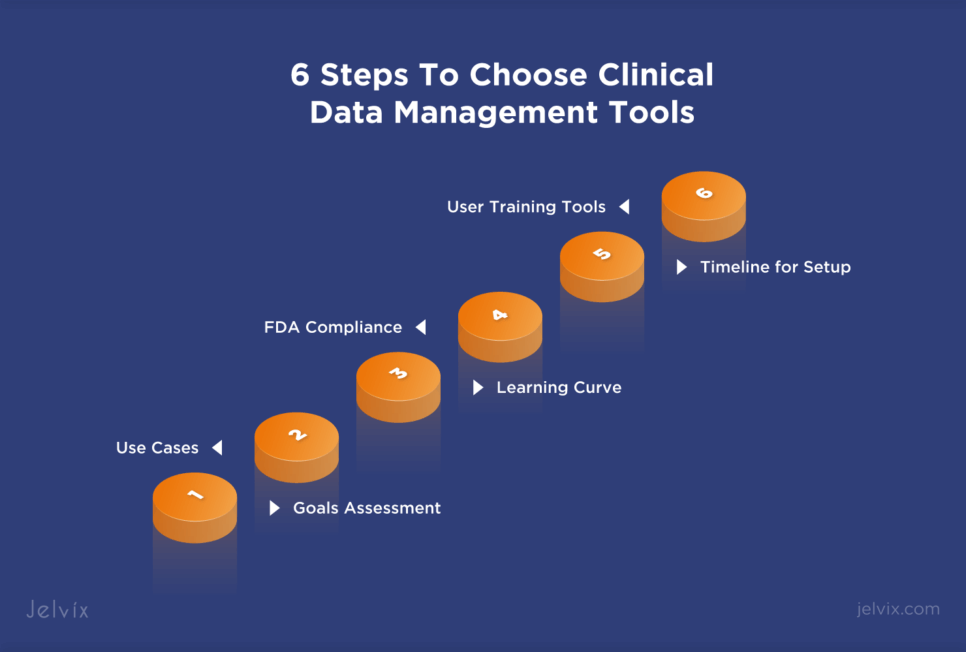
6 Steps To Choose Clinical Data Management Tools
As was noted earlier in this article, clinical data management tools play an important role in the success of clinical trials. The right tools help streamline data collection, ensure compliance, and enhance overall trial efficiency. The Jelvix team suggests that providers and vendors follow the key steps listed below to choose a tool that corresponds to their unique needs.
1. Goals Assessment
Before selecting a CTMS, take a moment to assess your systems’ current state and define your future goals. Understanding where you are right now and what you aim to achieve with a CTMS will guide your decision-making process and ensure the tool aligns with your objectives.
2. Use Cases
Start by evaluating CTMS use cases and requirements. Different clinical trials may demand different functionalities. Consider whether the tool can accommodate your study design, data collection methods, and data types effectively.
3. Learning Curve
Assess the learning curve associated with the tool. A user-friendly interface and intuitive features can significantly reduce training time and enhance user adoption. Opt for tools that offer a smooth onboarding process for your team.
4. FDA Compliance
Ensure that the data management tool aligns with FDA compliance standards and regulations. It should support data integrity, security, and audit trail capabilities to meet regulatory requirements. A tool with built-in compliance features simplifies the validation process.
5. Timeline for Setup and Implementation
Consider the time it will take to set up and implement the data management tool in your trial’s workflow. A tool that can be quickly deployed and easily integrated with existing systems, such as EHRs or telehealth solutions, will minimize disruptions to your study and accelerate the start of data collection.
6. User Training Tools
Evaluate the training resources and support provided by the vendor. Effective training tools and accessible customer support can significantly enhance user adoption and ensure that your team can leverage the full capabilities of the data management tool.
Conclusion
Expert Assistance in Developing Software for Clinical Trials
Clinical trial systems, including EDC, CTMS, IRT, and RTSM, enhance medical research transparency and security. When combined with a user-friendly interface, these can reduce operational costs, accelerate the pace of medical research, and improve its quality.
If you’re aimed at creating a flexible and scalable clinical data management system, consider collaborating with a software development partner. At Jelvix, our experienced developers specialize in crafting custom solutions that adhere to HIPAA compliance standards, covering data capture, patient engagement, and progress tracking.
To see the healthcare projects built by the team, take a look at our case studies. And if you would like to discuss the needs of your research team or institution, drop us a line!
Need a qualified team of developers?
Extend your development capacity with the dedicated team of professionals.