Today, as healthcare is rapidly digitizing, it provides more opportunities for stakeholders than ever before. The widespread adoption of intelligent medical devices, software applications, and cloud-based platforms has improved treatment and health monitoring in and out of healthcare settings.
However, healthcare still directly needs innovation. A shortage of doctors, disease outbreaks, and the need to cut supplier costs drive it to continue turning to health tech. And one of the latest trends in the industry, digital therapeutics (DTx), tries to solve these serious problems.
Jelvix is a pro in developing digital medicine solutions, and we are about to tell you about digital therapeutics and its potential for healthcare professionals, providers, and consumers.
Terminological Clarification
The term “digital” has recently been used a lot in today’s healthcare industry. Therefore, it is necessary to understand the difference between digital medicine, telehealth, digital health, and therapeutics. Despite the similarities in names, these digital health offshoots are different therapeutic practices and products. In addition, they all have clinical evidence requirements and special regulatory oversights. So, let’s dive into the details!
Digital Medicine
It is a narrower area of digital health that includes the use of technology-based products as tools to measure human health and support medical practice. Digital medicine, most times, deals with pharmaceuticals that join prescription drugs and sensors for ingestion. Hence, unlike digital health, such products often require regulatory certification.
Digital Health
It is a generic term encompassing everything – from electronic health records to wellness applications and clinically-validated therapeutic interventions that represent or improve existing personal therapies. Digital health contains other components of healthcare services and is an area of increased interest and investment.
Telehealth
This is the name for the practice of providing medical care over long distances using various technologies. Telehealth connects healthcare specialists and patients and can be part of the above practices, including digital therapy.
Digital Therapeutics
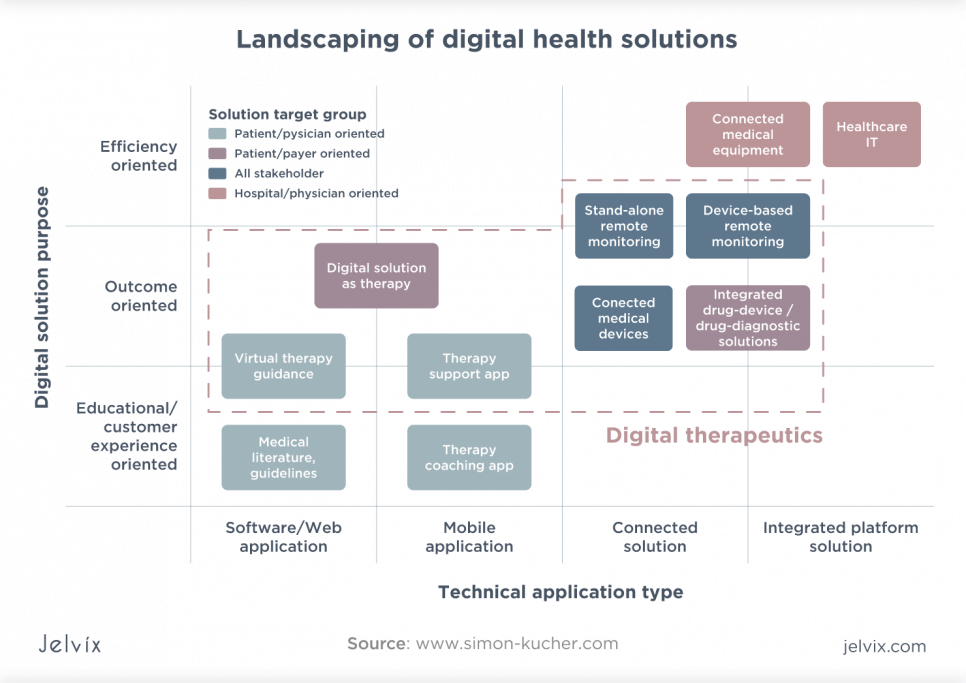
Also known as software as medicine, digital therapeutics is a new species of digital health – a set of digital tools or systems for the treatment and prevention of disease. Typically, these are software, mobile, or connected solutions, which fall into disease prevention apps, connected medical devices, and remote monitoring systems.
The digital therapeutics definition from the acclaimed non-profit organization Digital Therapeutics Alliance (DTxA) is “delivering healthcare services directly to patients through evidentiary, clinically validated software to control, prevent, and treat a broad range of diseases and disorders.”
It is necessary to differentiate digital therapeutic from digital pills, which are themselves physical, orally administered drugs (i.e., hardware) with built-in electronic circuits.
Digital therapeutic has several strengths. Firstly, it is the ability to manage lifestyle and support behavioral changes. The focus on patients’ well-being allows digital therapeutic tools to control diseases and lifestyle changes to maintain well-being and prevent disease. Digital therapeutic also provides information that we can use to improve personal care.
Secondly, many digital therapies are relatively inexpensive, making them an attractive and viable way to reduce healthcare costs. Introducing such non-invasive behavioral support methodologies is likely to become popular with patients, payers, and health care providers. Finally, they are more scalable than many traditional treatments.
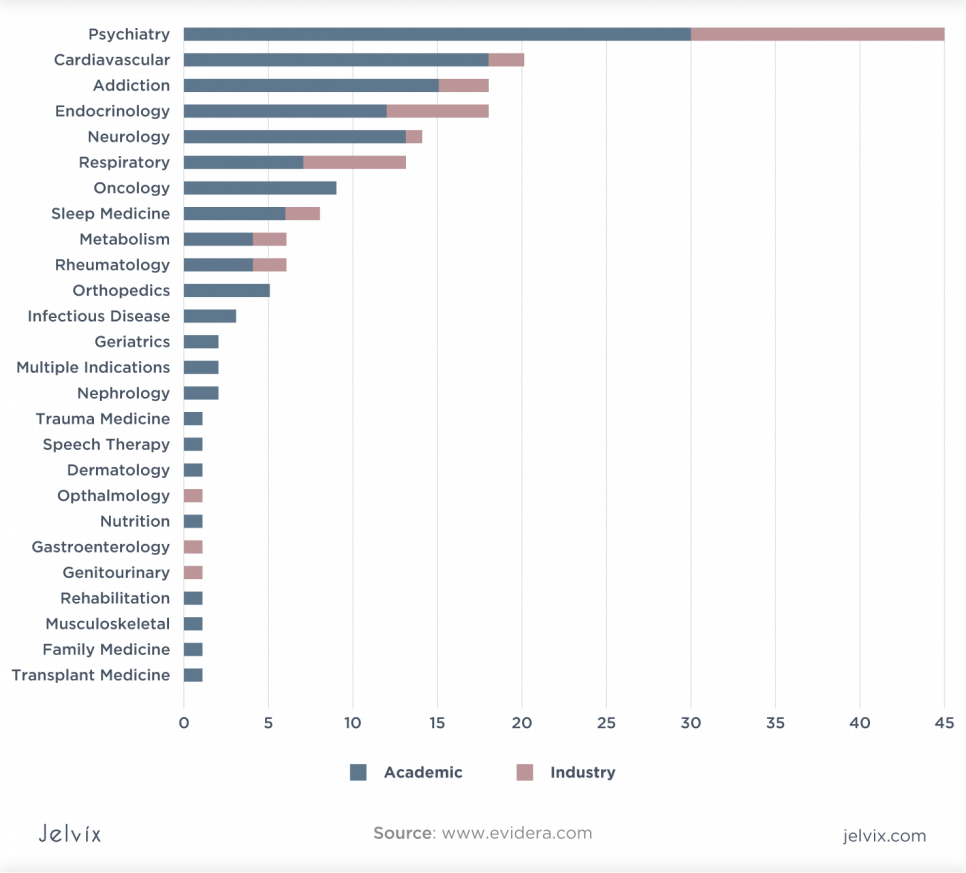
Virtually every healthcare sector is ready for DTx innovation. There has been significant growth in diabetes, asthma, drug addiction, behavioral and mental health, IDS, and obesity prevention.
Digital Therapeutic Tools
These are premium software systems designed to treat specific diseases and receive permission or approval from regulatory authorities. DTx products cover a broad range of disorders and illnesses and present clinically proven results that affect the condition. In addition, they help patients contend with various diseases and conditions through health-related advice, behavioral guidelines, exercise plans, medication alerts, and more.
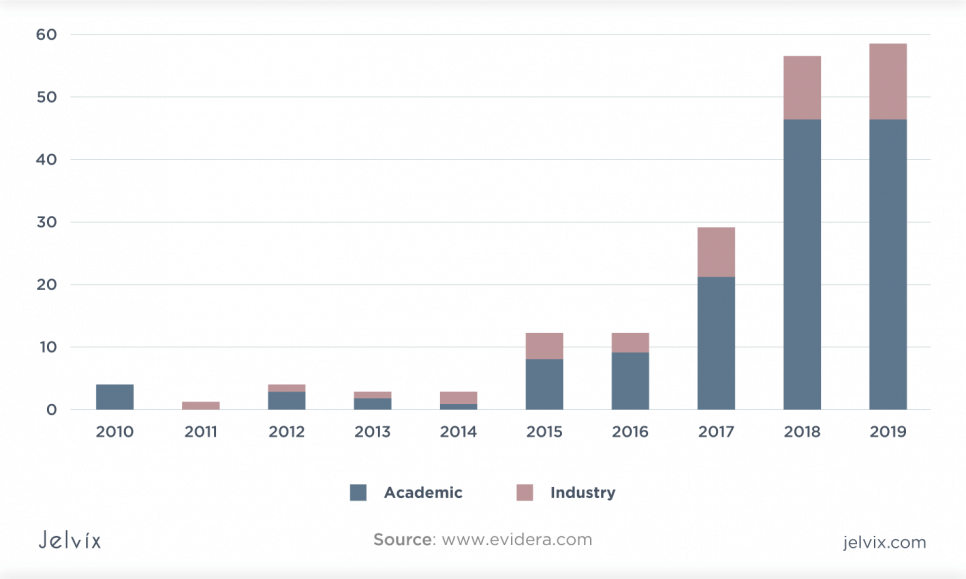
Although initially only the technology companies and academia showed interest in developing DTx, the possibility of using them in combination with drugs has sparked interest from large pharmaceutical companies, which have entered the DTx market through strategic partnerships with technology companies. Thus, the global digital therapy market will grow to $ 1.6 billion by 2027.
What Do Digital Therapeutics Do?
They complement and transform traditional healthcare delivery methods and allow to:
- Provide therapy using mobile phones, tablets, and similar technologies;
- Expand access to clinically safe and efficient treatments;
- Reduce the inconvenience of some aspects of traditional medicine by providing home care;
- Deliver meaningful results to patients and their physicians.
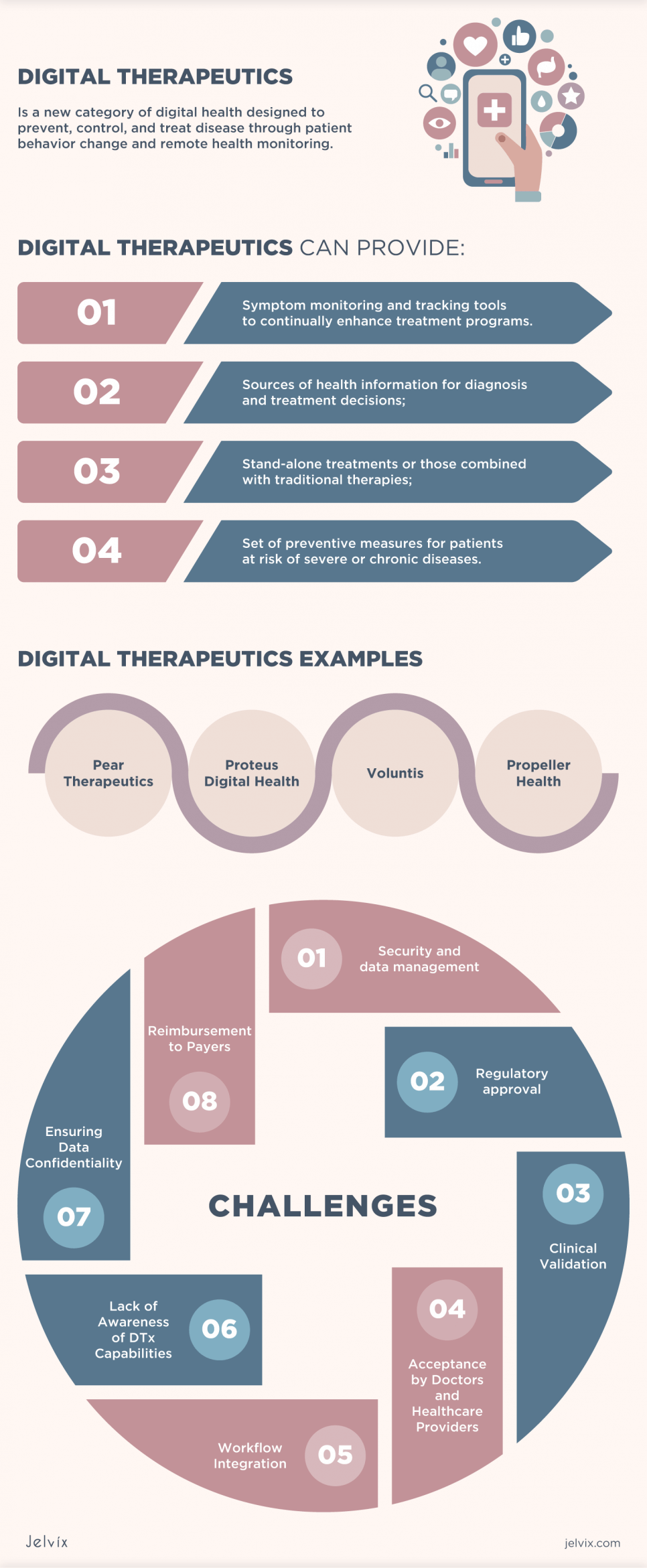
Also, digital therapeutics can provide:
- Symptom monitoring and tracking tools to continually enhance treatment programs.
- Sources of health information for diagnosis and treatment decisions;
- Stand-alone treatments or those combined with traditional therapies;
- Set of preventive measures for patients at risk of severe or chronic diseases.
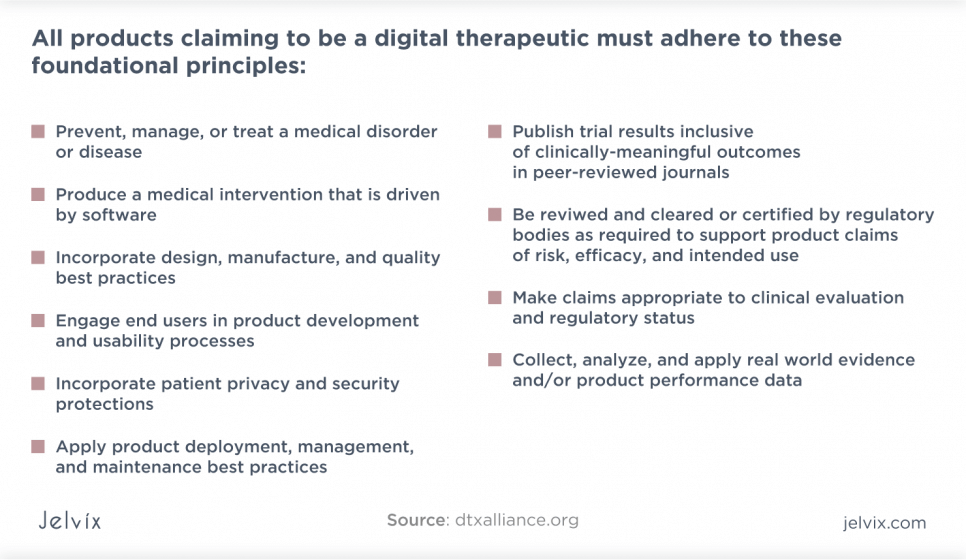
Digital Therapeutics Principles
The DTx has presented a list of ten DTx core principles that all digital therapeutic products must meet.
DTx Products Categories
Digital therapy products today can be divided into two broad categories:
- Software as a medical device serves one or more medical purposes without being part of medical hardware.
- A mobile medical application is a software adapted for mobile devices that are an accessory or addition to medical devices or turn into a medical device for diagnosing, monitoring, or treating diseases.
While all digital therapies align with industry core principles, these products can treat, manage, or prevent disease (which could mean improved health function).
DTx use cases
As already mentioned above, DTx is a new category of digital health designed to prevent, control, and treat disease through patient behavior change and remote health monitoring. Such solutions can induce patients to stick to a diet or medication regimen. And unlike conventional health tracking apps, which often target various conditions, digital therapy mainly focuses on a particular issue. Patients access digital treatment through a variety of software applications that can:
- provide helpful advice, such as first aid methods or instructions on how to cope with insomnia;
- adjust the medication prescribed by doctors, for instance, to treat asthma;
- promote behavior change through motivational and cognitive stimulation;
- gather and analyze patient data so that doctors can personalize treatment regimens;
- connect to various wearable or non-wearable medical devices to track and record vital signs.
Although DTx companies have mainly focused on behavioral and chronic conditions, some manufacturers have expanded their product range to acute conditions. For example, Click Therapeutics is already developing DTx to treat the acute coronary syndrome.
Digital Therapeutics Examples
Pear Therapeutics
The first digital therapy to receive FDA approval was reSET – an opioid addiction therapy program developed by Pear Therapeutics, a startup partnering with Sandoz. reSET is a Prescription Digital Therapeutic app that applies cognitive behavioral therapy to help patients cope with addiction.
Proteus Digital Health
The company has developed Proteus Discovery, a new system that measures the effectiveness of drug treatments, helps clinicians improve clinical outcomes and patients meet healthcare goals. It includes a vendor portal, a mobile app, and edible and wearable sensors.
Voluntis
The company built companion software for the healthcare sector, particularly for cancer, diabetes, and blood problems. As a result, Voluntis was the first digital therapy company to be listed on the stock exchange.
Propeller Health
The company’s digital platform has expanded patient adherence to treatment by up to 58%, reducing the use of emergency inhalers to 78% and reducing visits and hospitalizations for asthma and COPD to 57% and 35%, respectively.
Digital Therapeutics Benefits
It promises many benefits for physicians, healthcare providers, distributors, patients, and other stakeholders as a relatively new concept.
Patients gain access to proven technologies that are understandable, user-friendly, and improve health outcomes. In addition, they receive individual care and treatment programs, which are much more effective because of strict adherence to the therapy regimen.
Clinicians monitor the effectiveness of their treatment regimens and maintain statistics that can be useful for further adjusting the patient management plan.
Healthcare providers can monitor patients in real-time, take timely action, improve the efficiency of healthcare delivery, and reduce the demand for personal visits by remotely interacting with people with chronic conditions.
Payers cut their general medical expenses and insurance costs.
A McKinsey Consumer Health Insights study found that modern flexibility in insurance plans is an incentive for behavior change. Most said they would change their behavior – like playing more sports – to lower their insurance premiums.
Personalization is the cornerstone of DTx, meaning that it makes healthcare services personalized and tailored to each individual.
Learn about remote patient monitoring and why it be a vital tool for ongoing patient care that you can arrange efficiently and safely.
Major Challenges Facing Digital Therapy
Digital therapy companies need to consider four significant barriers that often accompany the adoption of digital health ecosystems and DTx in particular.
Security and data management
DTx transmits information over the Internet, so the risk of unauthorized access and manipulation of these solutions, or their underlying data, could jeopardize product confidence and patient care.
The FDA has defined two “tiers” for devices according to their perceived cybersecurity risk:
- Tier 1 (Higher Risk) devices are those that can be connected, wired or wireless, to other medical or non-medical products, networks, or the Internet. A cybersecurity breach involving such devices could cause harm to patients.
- Tier 2 (Standard Risk) devices are those that do not have the Tier 1 criterion.
Although the FDA has issued guidance on cybersecurity, labeling, and documentation for pre-marketing submissions, the agency does not currently require pre-market medical device safety audits to be completed. It is up to device manufacturers to define the level of cybersecurity risk associated with their products and take actions to minimize this risk. Then, the FDA evaluates the manufacturer’s design and risk management documentation for reasonable assurance of safety and efficacy.
In terms of data and control rights, manufacturers may use the Terms of Service, End User License Agreements, and Privacy Policies to establish and transfer company and user data rights to monitor, evaluate and share the collected data.
Regulatory approval
DTx is a completely new class of medicine and is quite diverse, so there is no universal basis for reviewers to evaluate and approve these products. The FDA, a global leader in addressing digital therapy challenges, has two options for placing a medical product on the market:
- FDA statement is granted when a product is like existing solutions on the market and does not pose a risk to consumers.
- FDA approval can be obtained if a product has passed rigorous clinical trials for efficacy and safety.
Today the FDA has become more flexible. One proof of this is the FDA’s Digital Health Software Pre-Certification Program, a pilot project to validate software-based medical devices. Realizing how slow it would be to approve each software version for a digital product, the FDA utilizes this program to support the developers. This way, product development can happen efficiently through rapid iteration rather than being stuck in a constant approval cycle.
With the ongoing COVID-19 pandemic, the FDA slightly loosened regulatory requirements to increase access to digital health platforms. And the European Medicines Agency has yet to develop a regulatory framework for the evaluation and commercialization of DTx.
Clinical Validation
Another critical step in DTx implementation is the need to undergo clinical validation that the products provide a trustworthy clinical outcome for the target clinical condition. Treatment efficacy is usually tested by comparing clinical results with the gold standard of treatment, no treatment, or the minimum standard of care.
The advantage of a digital therapy solution is that it can be tested in clinical trials and the market.
Acceptance by Doctors and Healthcare Providers
Doctors and providers should get familiar with digital therapy solutions before their mass adoption. They should review the results of clinical trials before recommending solutions to their patients. To do this, some DTx companies choose a pharmaceutical model in which partnerships with distributors are used to inform doctors about new solutions, clinical trial results, and decisions.
Workflow Integration
Any digital therapy tool that is poorly integrated into physician workflows will face obstacles in adoption. It applies to those tools that require clinicians to take additional steps to enter more information and those that need clinicians to abandon familiar applications. Systems that do not integrate with existing patient data are likely to be rejected. On the contrary, tools that help physicians correct even minor deficiencies in their daily tasks will be welcomed.
Lack of Awareness of DTx Capabilities
The field of digital therapy is little known to the market, and even with the ever-growing interest, many people are unaware of the types of DTx treatments that exist. That is another obstacle to the broader adoption of DTx solutions.
Ensuring Data Confidentiality
Digital therapy is based on large amounts of personalized patient data. Therefore, providers need to take care of data privacy and security so that DTx can safely enter the broader market. Also, DTx providers should ensure that their platforms can integrate with electronic health records or software that doctors already work with.
Reimbursement to Payers
The ultimate test of the value of digital therapy will be the amount the client is willing to pay. Payers choose to lower their healthcare costs by reducing emergency calls and complications or replacing actual doctor visits with automated software or virtual calls. A proven ability to perform any of these actions will increase the likelihood of digital therapy compensation.
The provider’s job is to get the attention of insurers and doctors and find working payment models. Companies currently partner with pharmaceutical companies and use traditional reimbursement and distribution methods or distribute them directly to patients through employers as side benefit programs.
Digital Therapeutics: Future Prospects
We’ve highlighted three considerations to keep in mind when designing and implementing your DTx capabilities.
Technologies
There are two promising areas associated with DTx technologies:
DTx development and curation platforms. They are essential for both speed-to-market and scalability. Implementing these platforms will help DTx companies focus on their core businesses (science) and allow them to take advantage of the built-in infrastructure and security features.
Innovations. The success of DTx solutions will largely depend on their ability to harness the power of new technologies as they approach maturation. Smart advisors, cognitive computing, biochips, efficient computing, tactile sensations, volumetric and holographic displays, and natural language questions are a few promising technologies that need a special place on the DTx roadmap.
Markets
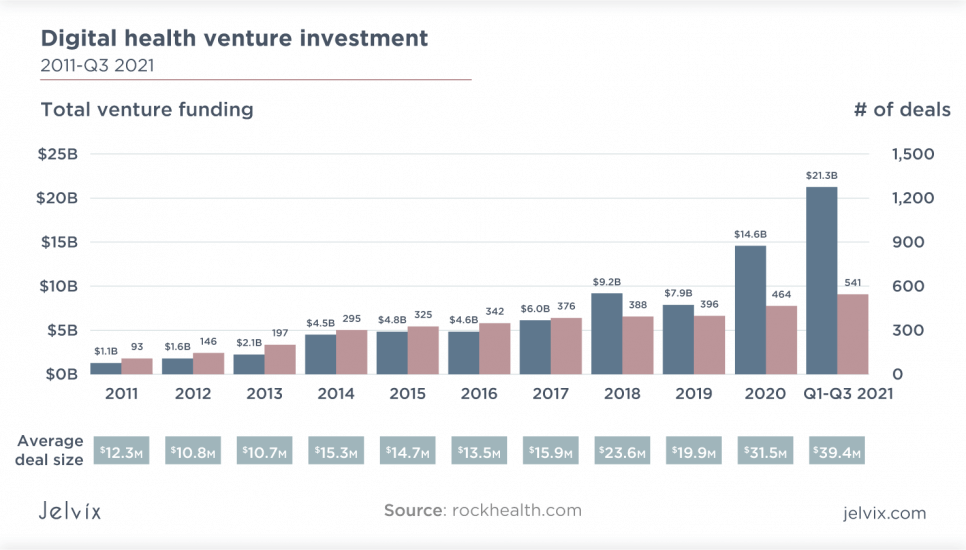
Digital health startups raised $21.3 billion in the first three quarters of 2021, which is the three most funded quarters in history, according to a Rock Health digital health venture capital report.
This level of investment underlines the growing demand for digital therapeutic platforms and tools across the entire healthcare ecosystem. As a result, Insider Intelligence has changed its forecast for 2025, and according to its calculations, the global capabilities of DTx will be not $9 billion as predicted before, but $56 billion. That is far ahead of any other digital predictions of the growth of medical technology.
Vendors
Successful digital therapy companies have capabilities that many pharmaceutical companies traditionally lack: advanced analytics, equipment design, human-centered product development, big data, and innovative, flexible business models. These capabilities may prove essential for pharmaceutical companies as healthcare transitions to a digital future.
Hence, pharmaceutical companies are likely to either actively cooperate with mature tech companies or buy DTx suppliers. It must be said that large mergers and acquisitions are key signs of market maturity and future growth.
Those in the market who hesitate to work with DTx are likely to miss out on tremendous opportunities. Pharmaceutical companies and medical device manufacturers that do not seize the chance to connect with DTx suppliers risk losing market shares to new competitors.
How to Start a Digital Therapeutics Project
Companies looking to get into the vanguard of medical innovation, called digital therapy, must consider every step, from product research and strategy outline to design and development. Deloitte describes two possible approaches:
- Digital use case. It is based on the idea of using certain digital possibilities as treatment. For example, you might decide to create a DTx solution that uses VR technology to recreate the environment and stimulate certain behaviors. It is promising but more difficult to implement because it requires a more focused approach to developing a solution and a larger investment.
- Therapeutic area. In this scenario, pharmaceutical companies and their MedTech partners decide which DTx product to create based on their clinical and therapeutic experience. They select a specific therapeutic area that requires new approaches or replace existing treatments.
In addition, DTx software development includes internal and licensing approaches within the company.
- In-house development. Large companies with sufficient financial resources can follow their product. But for this, they need to find and hire a team of professionals, which usually comprises:
- doctors and clinical trial design experts;
- programmers;
- product managers;
- data scientists;
- regulatory experts.
This process requires close cross-functional collaboration throughout the entire product development cycle.
- In-licensing development. This approach is optimal for newcomers to DTx or companies looking to expand their DTx portfolio without hiring additional labor. Instead of starting a project from scratch, they can invest in technological development or ongoing research by a third party.
This option is also suitable for academic laboratories and research centers that often lack the technology and skills to complete their research and bring it to market as patient-centered software.
DTx Product Development Steps
Regardless of the business strategy, DTx product development goes as follows:
Phase 1. Preclinical phase. It includes the development of a therapeutic intervention that will be digitized in the future.
Phase 2. A trial version of DTx software. It will allow you to assess the impact of therapy and the possibility of its further development. Specialists will also test the usability and accessibility of the solution for patients.
Phase 3. A pilot clinical study is usually performed on a narrow group of patients under the strict supervision of specialists.
Phase 4. Based on the pilot phase results, the product will either enter the full development stage, including technical and commercial aspects, or will be stopped. If the pilot is successful, the solution will undergo additional series of controlled clinical trials to prove its value to regulators, doctors, and insurance companies.
Final Thought
Everything moves fast when it comes to innovation. And we would like to see digital therapy become the standard of treatment, like prescribing drugs or treatment procedures. Most DTx technology solutions are mature enough to be made more affordable, functional, and patient-centered. AI, NLP, and ML have also reached the critical milestones needed to create large-scale digital therapy solutions.
Digital therapeutics represent a significant leap forward in value-based medicine, offering new treatment paradigms that are both innovative and effective. Jelvix stands at the forefront of this movement, providing Healthcare software development solutions that harness the potential of digital therapy to transform patient care.
So, all of this may well become a reality. Digital therapy has already proven its effectiveness in changing the behavior and lifestyle of patients suffering from chronic diseases and conditions. Soon, digital therapeutics will help transform the way patients are treated and encourage people to be more caring for their health.
As a reputable digital service provider, Jelvix’s mission is to grant you advanced digital therapeutic solutions. Therefore, if you are interested in the development and implementation of DTx, contact us right away!
Need a qualified team?
Extend your development capacity with the dedicated team of professionals.