We analyzed a range of hypertension and cardiovascular SaaS platforms, from corporate solutions such as Livongo and Omada to new RPM-focused tools, to understand where digital transformation solutions break and how top performers rebuild them. The report includes US and EU solutions at the consumer as well as enterprise levels, from remote monitoring and analytics to clinical workflow systems.
All findings are based solely on publicly available sources, including API documentation, interoperability models, and performance reports. No personal or proprietary data was accessed.
Why this matters: Despite a market exceeding $3 billion according to Grand View Research, growth remains throttled by fragmented architectures. In practice, APIs frequently do not normalize data; FHIR adoption is fragmented and stops at the sandbox level; and lack of interoperability continues to stymie real-time care coordination. The potential of connected care slips away when hypertension management software generates data more rapidly than it can make sense of or protect.
As a result, our analysis identified a recurring pattern: top platforms, regardless of asset variety, viewed integration as a plan, not just plumbing. They treat data architecture and compliance as a single continuum, and analytics focus on raw telemetry to generate clinical intelligence. Everything indicates that it is no longer a matter of choice but a question of the competitiveness of unity.
The findings establish a new standard for digital-health leaders. We present what can reshape your roadmap: treating integration as a product discipline, embedding compliance in the architecture, and using AI as connective tissue.
The Data Dilemma in Digital Hypertension Care: Rise of Remote Blood Pressure Monitoring
Digital hypertension care finds itself at a curious intersection where we’ve got billions of data points collected from connected devices, yet true comprehension is much further down the road. Though a small slice of the telehealth pie, remote blood pressure monitoring has been elevated to a touchstone in a rapidly expanding $10 billion marketplace structured around wearables, cloud analytics, and EHR integrations.
According to Fortune Business Insights, the market for digital blood pressure monitors alone was valued at around $1.08 billion in 2024 and is expected to roughly double by 2032, driven by smarter algorithms, app-based tracking, and the shift away from outdated mercury devices.
North America is ahead with over 40% share of the global market, spurred by coordinated reimbursement coverage and quick launches from top 10 cardiovascular medical device companies, including Omron, Philips, and GE Healthcare. Japan and Europe are experiencing the same thrust, from sensor miniaturization to predictive telemetry. But they still run into the usual suspects:
- mismatched data formats;
- untrustworthy timestamps;
- low FHIR integration.
Underneath the excitement in the market is a more profound problem—of architecture, not ambition. The collective feed of readings from endless monitors and wearables pours through siloed software developer kits into oceans of information (data lakes) that converse in different digital dialects. What is often marketed as “real-time intelligence” is, in fact, late data concatenated by rule-based scripts. Digital hypertension care might degrade into a disconnected mess of sensors without true interoperability. The real leaders will be those who can transform raw signals into actionable insight.
Hypertension Management Software: Analytical Framework and Data Provenance
The analysis used a documentation-first method to track how leading hypertension management software handles data integration. The team reviewed public datasets, API blueprints, and developer sandboxes from platforms like Hello Heart, Omada Health, Livongo, Aktiia, and Withings to map the interoperability stack and pinpoint failure points. More specifically, the research was trying to expose how ingestion pipelines collapse into a long-tail digital health integration friction, and ultimately, why hypertension apps fail to scale.
The express focus was on platforms in their growth phase that share a public API and claim EHR interoperability. No proprietary information was used in generating the dataset. The totality of the research was built on verifiable sources, which included HL7, ONC, and HIMSS frameworks only, while being blind to speculative vendor claims.
As a result, findings established the connection between financial maturity and integration readiness, showing that the weak SDK alignment and poor FHIR adoption disrupt the operational core of the digital hypertension ecosystem. Therefore, the strongest platforms succeeded by transforming API transparency into an interoperable intelligence.
Looking to stay ahead of the curve? Discover how predictive analytics solutions can drive smarter decisions and better outcomes.
Where Data Integration Fails
As digital hypertension platforms expand, a critical weakness keeps surfacing: data moves easily from devices to the cloud, but rarely reaches clinical insight intact. In healthcare software development, this gap between collection and comprehension exposes the core issue: most architectures were built for storage with no room for intelligence.
API and Device Fragmentation in Health Data Analytics
The ingestion stack in particular often feels improvised among today’s isolated blood-pressure ecosystems. Every layer creates friction where it should be plain sailing, data flow.
One vendor might transmit the time stamped as a Unix epoch with a zone offset, and another converted the payload to ISO 8601, but now without any context. At other times, the duplication grows weirder. Say, two distinct systolic readings show up under different field names, both taken as truth until a parser breaks. On average, platforms juggle about 4.3 integration layers before reaching normalization. And each one dilutes fidelity a bit more.
Key Issues with Data Fragmentation:
- Inconsistent formatting: Timestamps differ across APIs (Unix vs. ISO), resulting in synchronization errors.
- Duplicate fields: The same metric (e.g., systolic pressure) can appear twice under separate field names, causing data conflicts.
- Missing metadata: Context such as device type or measurement conditions is often stripped during ingestion.
- Drifting units: Measurement standards differ, complicating analytics and clinical interpretation.
This culture of fragmentation inflicts its damage silently. Every new firmware version or line of newly acquired devices resets the adapter cycle—refactoring shit, re-testing the ingestion for… some reason, rewriting more mapping logic that should have been abstracted out years ago. This is a systemic tax on scaling up.
SMART on FHIR: The Production Integration Gap
In many hypertension-platform architectures, the leap from SDK to EHR fails precisely at the juncture of the FHIR interoperability standard. Even if systems say they have transitioned from SOAP to REST or are “FHIR ready,” few mature vendors have achieved a production-level integration with systems such as Epic or Cerner. A survey published in JAMIA found a very high proportion of digital health platforms (up to 70%) assert support for FHIR APIs, compared to a tiny fraction (<30%) implementing full SMART-capable launches in enterprise EHRs, as stated in the latest reports.
The framework SMART on FHIR builds upon FHIR, providing app-launch context, OAuth2-based trust, and encounter-aware workflows. However, most of the platforms cover only token exchange and very simplified read / write operations for the software design of hypertension management. The point is, true clinical workflow integration requires something extra:
- active patient context;
- encounter-related metadata;
- role-specific rights to perform order-request actions, including error prevention;
- dynamic access to external data.
Without that, the gear for remote blood-pressure monitoring goes unused.
Data Quality and Usability Gaps
Driven by speed-to-market pressures, many hypertension management platforms bypass critical real-time data validation and normalization. Consequently, the vast data streams collected are often unreliable for clinical use, leading to a torrent of unmanageable, clinically untrustworthy data.
The architecture of dysfunction:
- Some platforms still rely on manual CSV uploads, triggered by human schedulers.
- Others use continuous sync but fail to verify semantic alignment, allowing readings to drift without context.
Both architectures collapse in opposite ways: one starves analytics, the other corrupts it.
For AI-driven hypertension monitoring tools, such an inconsistency is fatal. Algorithms that have learned from non-standardized units and fluctuating timestamps inherit the same noise they were meant to read. What follows is a simulation of intelligence. The model performs well until it meets reality, where corrupted inputs cascade into false predictions and erode clinical confidence.
Specialists in AI software development often patch the gaps with ad-hoc preprocessing scripts or heuristic filters. It buys time but destroys trust. You can normalize on your systolic values or even impute missing metadata. But without architectural coherence, the pipeline topples over at scale.
Compliance Blind Spots
In the architecture of digital platforms for treating hypertension, the facade of clinical interaction often hides deep gaps. In many cases, fundamental mechanisms such as at-rest encryption, token rotation for OAuth flows, or immutable data provenance logs are missing. The absence of these controls undermines the trust of enterprise partners and creates barriers to embedding workflows into hospital systems.
While telemetry metadata is generally preserved, the “last mile” of compliance, data presented as traceable, easily audited workflows within the EHR, frequently collapses. Few systems maintain granular audit trails that denote who accessed something, when, and how it was altered. Without this lineage, platforms fall short of the standards required by clinical interoperability frameworks. Regulatory bodies increasingly emphasize end-to-end traceability, where every data transformation must be linked back to its source.
The cost of non-compliance in this area is significant. Platforms that do not have robust audit control mechanisms may be blocked from partnering with large provider systems or face retrospective remediation under the watchful eye of regulators.
Scaling Bottlenecks
Most API integrations in healthcare problems are related to three interrelated malfunctions.
First, a huge single API endpoint handles input, normalization, and routing. There is no horizontal partitioning, queuing, or traffic shaping. When thousands of wearable cuffs synchronize simultaneously, latency increases and data becomes stale.
Second, input rate limits or token quotas from EHR systems or national health services restrict access to the back end.
Third, the conversion logic is still monolithically embedded in the same API stream. However, it should be offloaded to streaming processors or microservices. The result: when the number of devices doubles, CPU and memory latency often more than double as well, leading to buffering, timeouts, and user disconnections.
To draw the line here, architectural resilience defines scalability. The persistent integration failures in hypertension platforms stem from architectural decisions. Systems designed only for data storage will always struggle to generate intelligence or sustain clinical relevance.
Data integrity must precede analytics. Real-time validation and normalization are preconditions for trustworthy insights and effective AI performance. Analytics devolves into speculation without structured coherence at ingestion.
And finally, compliance maturity drives market access. In an ecosystem where interoperability equals credibility, auditability and lineage tracking are competitive differentiators.
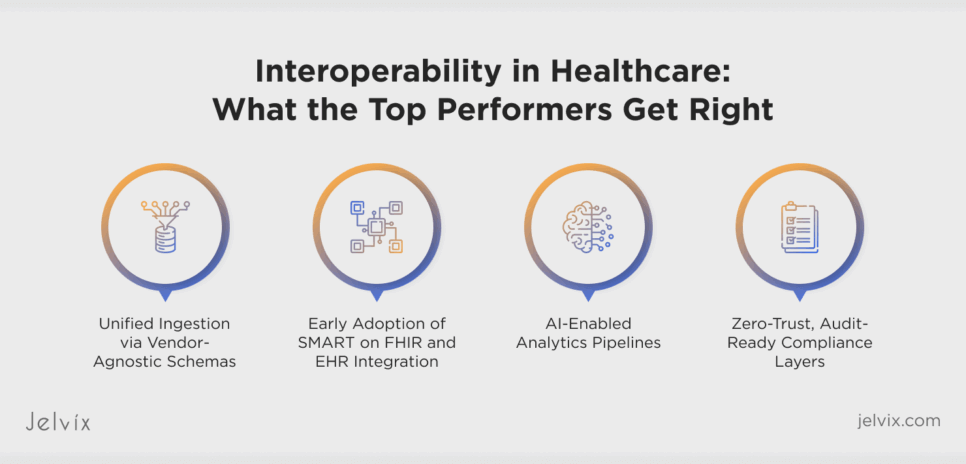
Interoperability in Healthcare: What the Top Performers Get Right
When studying the best platforms in their field, a clear archetype emerges: systems that move from simple data accumulation to fully integrated architectures based on analytical data. The difference lies in how these performers organize collection, processing, analysis, security, and product strategy into a unified concept.
Unified Ingestion via Vendor-Agnostic Schemas
Livongo (now part of Teladoc Health) demonstrates how a scalable data ingestion architecture becomes a strategic asset. The company’s system supports devices from different manufacturers (Omron, A&D, iHealth, and others) thanks to vendor-independent schemas built on normalized data contracts and dynamic mapping tables.
Instead of writing adapters for each device, Livongo’s data collection layer abstracts input data into a canonical JSON schema before conversion. This approach allows data to follow a single path from the patient’s device to the analytics engine. This model reduces engineering costs, simplifies device certification, and minimizes latency spikes during firmware or SDK updates.
Explore how top platforms master software development roles to fix data integration gaps and accelerate outcomes.
Early Adoption of SMART on FHIR and EHR Integration
Omada Health’s architecture sets the standard for how to improve interoperability in healthcare through deep EHR integration. Instead of using FHIR endpoints as data-transfer bridges, Omada implements SMART on FHIR to enable workflows.
The system uses OAuth2 and OpenID Connect to manage secure launches from the provider’s EHR interface. This allows healthcare teams to access Omada dashboards without leaving their workspace. Such an “embedded interaction” transforms external digital health data into first-class clinical data: visible, usable, and trackable within the provider’s core workflow.
AI-Enabled Analytics Pipelines
The true measure of maturity in the field of SaaS for treating hypertension lies in the transition from data visualization to data interpretation. Livongo and Hello Heart use AI-based hypertension monitoring tools, setting an example for others to follow.
Their pipelines integrate machine learning models that detect micro-patterns in pressure fluctuations and correlate them with important influencing factors. The architecture supports real-time conclusions, where new indicators trigger predictive prompts or escalation workflows for at-risk users.
Furthermore, by integrating predictive analytics directly with EHR systems, these tools can automatically generate clinical decision support (CDS) prompts, bridging the final gap between consumer health data and provider action.
Zero-Trust, Audit-Ready Compliance Layers
Leading hypertension treatment platforms are developing their security systems as dynamic ecosystems. They use zero-trust architecture, which means that every data transaction must undergo authentication and identity verification, even within the internal network.
From a technical standpoint, this includes:
- multi-layered encryption;
- rolling token systems;
- event-based audit logs;
- continuous anomaly detection in API calls.
Every PHI event is logged with complete origin metadata: source, transformation, time, and destination. This trail is immutable. It is often stored in event stream processors such as Kafka or in blockchain-based audit repositories to ensure forensic-grade traceability. This rigor not only reduces the risk of non-compliance but also speeds up contracting with enterprises.
The final differentiator is philosophical. The best performers treat integration as product DNA. For them, clinical interoperability is baked into every stage of development.
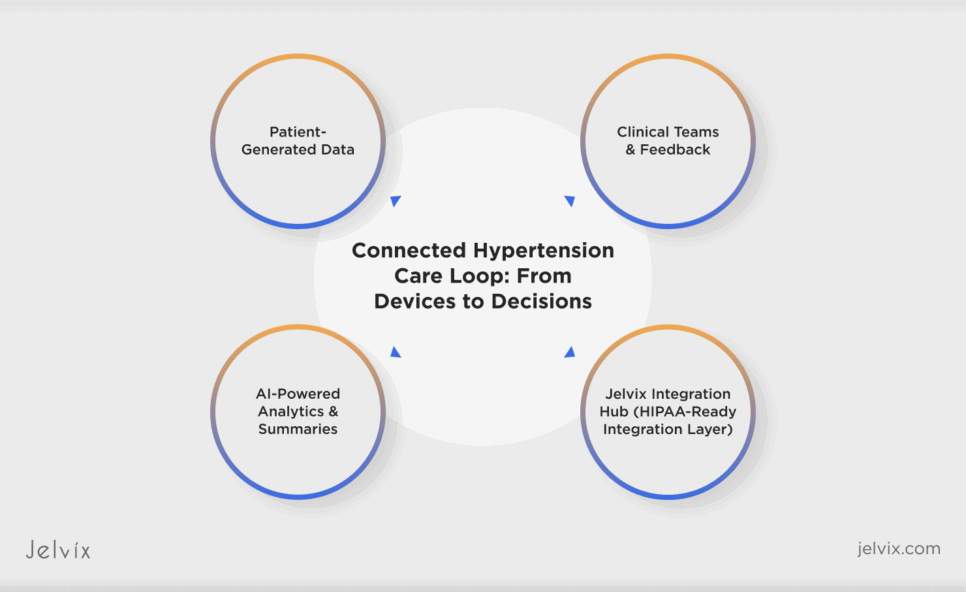
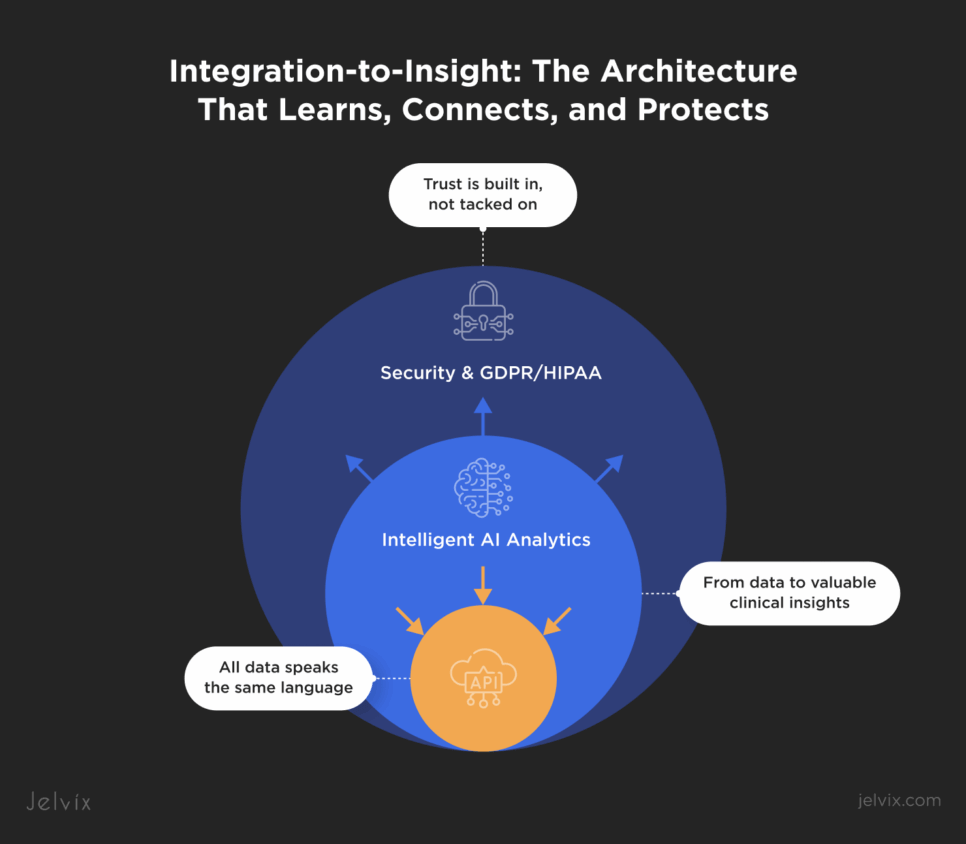
Digital Health Integration: The Jelvix Integration-to-Insight Framework
The fragmentation plaguing hypertension platforms didn’t occur by accident. It is the direct result of treating integration as infrastructure rather than intelligence. Jelvix redefines that paradigm. Our relentless approach to integration sees it as not just a pipeline to maintain but a living ecosystem where every data point must travel through a secure, intelligent, and compatible channel before it can become clinically meaningful.
What we call the Integration-to-Insight Framework it’s an adaptable architecture strategy refined across multiple digital health platforms. At the center of this ecosystem is a continuous cycle: data connects, learns, and protects. Integration bridges centrally unify device telemetry and EHR streams under a shared data language that erases translation friction. AI-driven analytics converts the stabilized input to predictive intelligence that informs both care teams and product strategy. And compliance-by-design frameworks safeguard every transaction through HIPAA- and GDPR-aligned controls, where traceability and zero-trust architecture define credibility.
This integrated design lets digital health systems scale with purpose. In one recent engagement, a hypertension care platform refactored its data infrastructure using this model, which cut integration time by 70% and cleared an enterprise audit in 14 days. It wasn’t just speed anymore; it was structural clarity. When integration behaves like a design principle and not an afterthought, the data no longer loops in circles as direction and intelligence guide it.
Strategic Recommendations for Digital-Health Leaders
Technology only creates value when leadership understands where to aim it. In digital hypertension management, that aspiration is set out in our architectural intent: creating systems that adjust and evolve without collapsing under the weight of their own complexity.
- Treat interoperability as an ecosystem.
Leaders must invest in integration as a strategic differentiator. A platform that brings together device data, clinical records, and analytics under a single, coherent data model reduces friction, laying the foundation for product speed and corporate trust.
- Build intelligence into the pipeline
Insight is rooted in accurate, sensitive data. When AI models train on structured, validated data, they expand clinical reasoning rather than replace it with imitation. Jelvix’s expertise in AI analytics demonstrates how clarity at the data layer multiplies impact at the insight layer.
- Design compliance as experience.
Regulatory frameworks like HIPAA and GDPR shouldn’t slow progress and shouldn’t be treated as walls to break. They should shape resilient systems. Embedding traceability and access control directly into the architecture ensures enterprise readiness from the first deployment.
- Measure scalability through resilience.
Sustainable platforms scale by design. Elastic infrastructure, self-healing APIs, and modular orchestration enable continuous growth without degradation in performance or security.
The next generation doesn’t compete on features; most importantly, it integrates, reasons, and protects better. The sector’s most successful players will view the architecture not as a backend system but as a continuous loop of intelligence.
Conclusion: The Future of Connected Hypertension Platforms
The new wave of hypertension platforms is evolving into a living ecosystem, one that integrates and learns while protecting the integrity of every data flow. The architecture of connected care is changing fast: predictive models are now implemented on FHIR-native grounds, telemetry arrives as verified clinical data, and authorization starts with identity-bound SMART on FHIR sessions. Regulatory-grade validation is not a matter of preference but a requirement.
Precision now depends on computation and even on context. Personalization devices of the future will need to incorporate information about our physiology and social contexts, both of which signal outcomes far beyond a simple blood pressure measurement. Systems need automation in force and the scope of care. Systems that consolidate the best of automation and medical human judgment achieve far greater adherence and deeper levels of trust by balancing the consistency necessary for effective care with compassion.
For AI systems to grow responsibly, they need structure and visibility. Bias checks must operate within the CI/CD pipeline, and lineage logs must remain immutable through every update. Systems that ensure auditability and observability create the transparency required for enterprise and clinical adoption alike.
There is one simple, closing truth easy enough to guide a roadmap: the true competitive advantage lies not in gathering data, but in connecting it responsibly and intelligently. You build for the live clinical context, for verifiable lineage, and for models that can grow and learn without drifting, and you stop having a dashboarding tool and start building care.
If your platform faces similar challenges, book a consultation with Jelvix experts to explore how this architectural pattern can adapt to your system’s scale. Our engineers and strategists can help translate fragmented infrastructure into interoperable intelligence.
Struggling with fragmented data in your hypertension platform?
Partner with Jelvix to bridge integration gaps and elevate care delivery.